Kinetics and Mechanisms of Emulsion Polymerization
Emulsion polymerization is one of the most important polymerization techniques in the industry and laboratory used for the production of a large number of polymers and copolymers in form of latices which are utilized in various fields, including coatings, paints, inks, sealants, adhesives, and rubbers. Although emulsion polymerization techniques have been used on an industrial scale since the 1930s, only in the late 1940s a theoretical description has been suggested for the processes and the qualitative or quantitative reaction rates. The first qualitative mechanism for the emulsion polymerization was suggested by Harkins in 1945.1,2 He postulated that the main locus of chain growth can be found in the stabilized polymer particles rather than in the large monomer droplets. Smith and Ewart developed later a quantitative model based on Harkins assumptions.3,4
The polymerization process can be divided into three stages distinguished by their reaction rate over time.a The first stage is the so-called particle nucleation; the addition of initiator to the emulsion produces free radicals in the aqueous phase. Some of these radicals diffuse into monomer-swollen micelles and initiate the polymerization. Thus, the micelles are the locus of polymerization. Both the reaction rate and the number of particles increases steadily during this stage. The second stage is reached when the reaction rate and the number of particles assumes its maximum value which remain more or less constant throughout this stage. The reaction mixture consists solely of monomer swollen polymer particles with dissolved monomers. Both the monomer concentration and the reaction rate steadily decrease with time and stops when monomers have been used up.
Stages of an Emulsion Polymerization
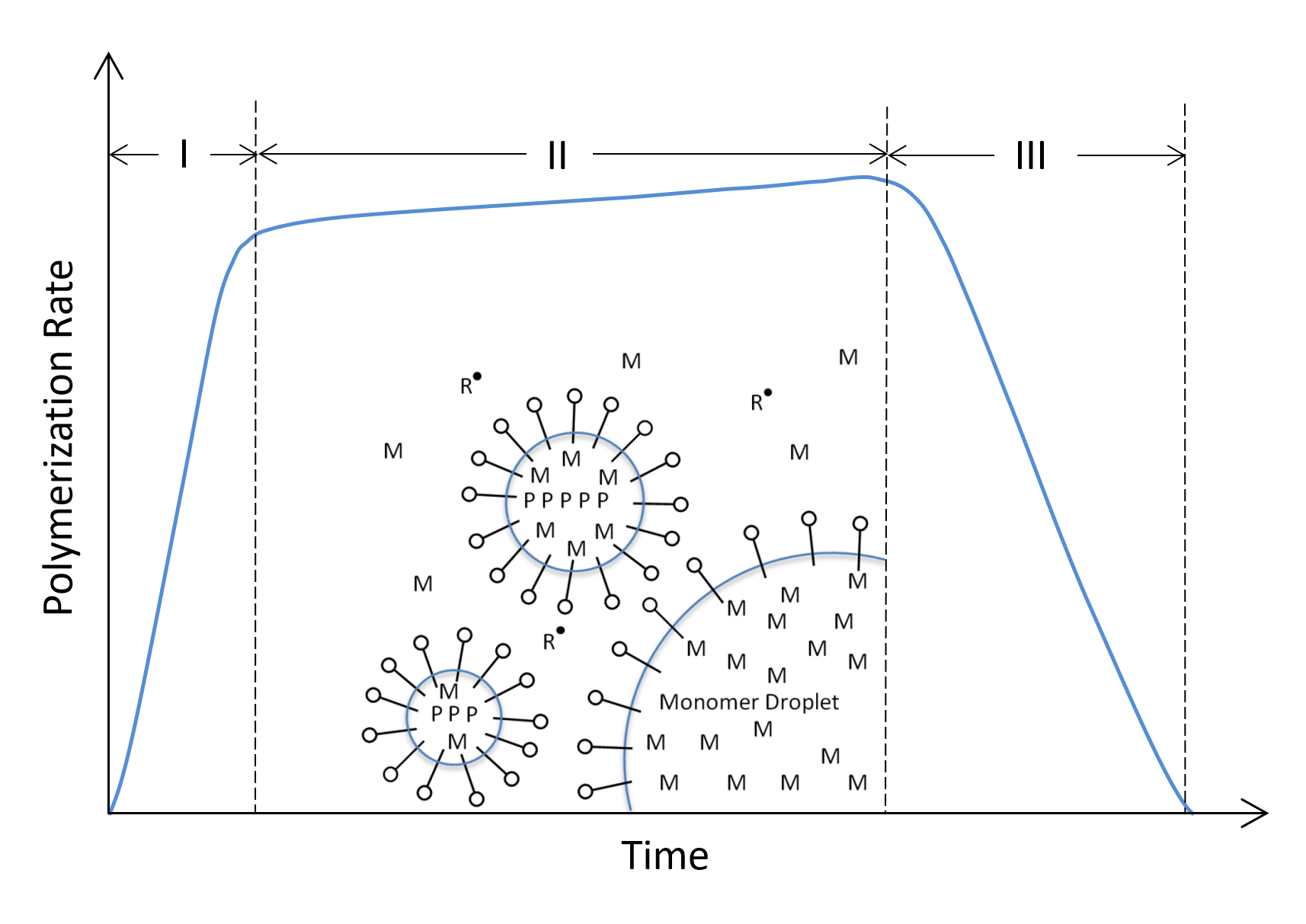
Assuming steady-state conditions, and assuming the reaction rate is independent of the particle size and polymer molecular weight, the overall rate of polymerization, Rp, at any time is given by the product of the concentration of active latex particles, Np, and the average concentration of monomer, [M]p, in the latex particles:
Rp = kp Np [M]p
Np can be expressed as the product of concentration of polymer particles and the average number of radicals per particle:
Np = n NT / NA
where n is the average number of radicals per particle, NT is the number of monomer-swollen particles per unit volume of continuous phase and NA is the Avogadro number. Combining the two equations yields the rate of polymerization as
Rp = kp n NT [M]p / NA
n NT is zero at the beginning of the polymerization since n is zero. In a polymerization batch experiment, it is more convenient to measure the fractional conversion of monomer into polymer, f:6
df/dt = kp n NT [M]p / nM,0 NA
where nM,0 is the initial added number of moles of monomer per unit volume of continuous phase. NT decreases whereas n and n NT increases with time during stage I. At the start of stage II, NT reaches a steady state value N and remains constant throughout the course of polymerization (stage II & III). The average number of radicals per particle, n, and the velocity coefficient, kp, may not reach a constant value but may vary with conversion of monomer to polymer.
References & Notes
W.D. Harkins, J. Chem. Phys. 13, 381 (1945); 14, 47 (1946)
W. D. Harkins, J. Am. Chem. Soc., 69, 1428 (1947).
W.V. Smith, and R. H. Ewart, J. Chem. Phys. 16, 592 (1948)
W.V. Smith, J. Am. Chem. Soc. 71, 4077 (1949)
S.C. Thickett, R.G. Gilbert, Polymer 48, 6965 - 6991 (2007)
Q. Wang, S. Fu, and T. Yu, Prog. Polym. Sci., Vol. 19, 703 - 753 (1994)